
Five Methods of Testing Disinfection Efficacy in the Real World
Part 1 of this series explains the real-world conditions that affect disinfectant efficacy: variable conditions including type of applicator (e.g. sprayer versus fogger), dwell times, soil load, surface types, humidity, temperature, etc. So, in a real-world setting, what are the options to test pathogen reduction efficacy of a disinfectant in a specific use case? This section describes five efficacy test methods that are commonly used, and lists some of the tradeoffs between them. The five methods being compared include: adenosine triphosphate (ATP), incubated ATP, chemical strips, electrochemical sensors, and microbial cultures.
1. Adenosine Triphosphate (ATP) Testing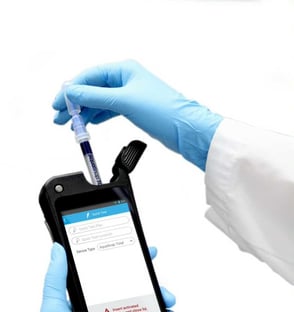
ATP is an enzyme that is present in all living cells, and an ATP monitoring system can detect the amount of organic matter that remains after cleaning. An ATP test procedure consists of these steps: (1) swabbing a surface, (2) immersing the swab in a test solution, and (3) measuring the bioluminescence from the immersed swab in a Luminometer. The quantity of light generated is directly related to the amount of ATP in the sample, which indicates surface cleanliness.
Pros:
- ATP testing is relatively easy to perform, can be accomplished in about 60 seconds, and provides immediate data on the status of cleaning activities.
Cons:
- Both living and recently killed organisms create ATP on surfaces and there is no known correlation between ATP level and numbers of live pathogens on a surface. Consequently, ATP testing does NOT offer data on disinfection thoroughness. In practice, ATP testing is used to determine good/bad cleaning status.
- ATP is not produced within some microbes, most notably viruses, and ATP-based systems will not detect the presence of these organisms.
2. Incubated ATP Testing
ATP testing can be improved by adding an intermediate incubation step between steps (2) and (3) above in the ATP test procedure description. During this additional step, the sample swab is immersed in an enrichment broth and placed within an incubator for up to 8 hours so that the live organisms will multiply and minimize the measurement impact of recently killed organisms. The bioluminescence measurement method is essentially the same as the non-incubated ATP test above.
Pros:
- This type of testing is similar to ATP testing in ease of performing.
- Because only live organisms grow during the incubation step, this process is used for same-day tests to evaluate surface disinfection.
Cons:
- This test has large measurement uncertainty because results are very sensitive to variables such as culture time, organism types, and swabbing process.
- Results are very sensitive to culture time (e.g. a 10X growth in one hour), and it takes some time to process a swab. Consequently, it is difficult to process many swabs within the necessary time window to provide an accurate measure of pathogen log kill. In practice, this process is used to determine good/bad disinfection status.
- Like ATP testing, incubated ATP testing will not detect the presence of some microbes, most notably viruses.
3. Microbial Culture Testing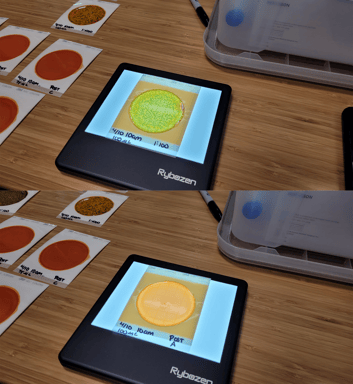
Traditional microbial culturing is the precision process used in microbiology laboratories for measuring pathogen log reduction. This process can be used outside of a laboratory with non-pathogenic organisms such as Escherichia coli K-12 (E. coli K-12). The testing includes spreading a pathogen broth on tiles, with some tiles to be treated with disinfectant and other untreated tiles used for comparison. After some tiles are exposed to the disinfectant, all tiles are swabbed, plated, incubated for up to 48 hours at elevated temperature. After incubation, bacterial colony forming units (CFUs) are counted for both the samples treated with disinfectant and the untreated samples. The untreated samples are typically diluted with a number of 10:1 dilutions (e.g. 1000:1 and 10000:1) before plating to maintain the total CFUs on a plate to <300 for accurate counting. When complete, the CFU counts for treated and untreated samples are compared to accurately compute pathogen log kill.
Pros:
- This process directly measures microorganism CFUs to calculate pathogen reduction.
- It is a scientifically verifiable and repeatable process, and can characterize very high log reductions, limited only by the total pathogen counts in original samples.
- Within a microbiology laboratory, a large variety of pathogens can be tested.
Cons:
- It is a time-consuming process, with many steps including incubation that can take up to 48 hours
- The process requires relatively expensive test equipment and requires microorganism test samples that have relatively short shelf lives.
- Outside of a controlled microbiology laboratory, only a few non-pathogenic microorganisms can be tested safely.
- Microbial culturing is used for bacterial testing and a completely different process is needed for viruses.
4. Chemical Test Strips
Chemical test strips are used to test for the presence of specific chemicals. Strips are available for various chemicals used within disinfectants (quats, bleach, hydrogen peroxide, etc.). Strips provide a rapid test, typically using a color change process.
Pros:
- Tests are easy to perform, and results are returned very quickly.
- Strips are inexpensive and readily available for various chemicals.
- This is a common test method used for chemical detection in many industries.
Cons:
- The color change interpretation adds measurement uncertainty and error.
- This process does not measure actual reduction in microbes. The chemical concentration must be correlated to pathogen log kill with other tests.
5. Electrochemical Sensors
Like test strips, electrochemical sensors are used to test for the presence of specific chemicals. Sensors are available for various chemicals used within disinfectants and provide real time measurements. Most electrochemical sensors are calibrated and provide a quantitative measurement of the chemical concentration.
Pros:
- Tests are easy to perform, and results are returned in real time.
- Sensors return actual measurement values for the chemical concentrations.
- Sensors are available for various chemicals.
Cons:
- Sensors may be relatively expensive.
- Electrochemical sensors may degrade over time and may be cross sensitive to other chemicals. Both issues can cause measurement errors if the user is not careful.
- This process does not measure actual reduction in microbes. The chemical concentration must be correlated to pathogen log kill with other tests.
- Electrochemical sensors measure chemical vapors (gas phase) which is a portion of the total aerosolized fog that also includes small droplets (liquid phase), providing an indirect dosage measurement that can be affected by environmental conditions such as humidity.
At Build With Robots (BWR), we have used all five test methods described above within different situations to better understand and validate the use cases for our aerosolized hydrogen peroxide (aHP) disinfection solutions. Continue reading Part 3 for an example of applying these tests to measure and validate disinfection efficacy in real-world conditions.
Part 1: Breezy BioCare RTU: Why Real-World Efficacy Testing is Needed
Part 2: Five Methods of Testing Disinfection Efficacy in the Real World
Part 3: Testing Aerosolized Hydrogen Peroxide Disinfection Efficacy in Real World Conditions



